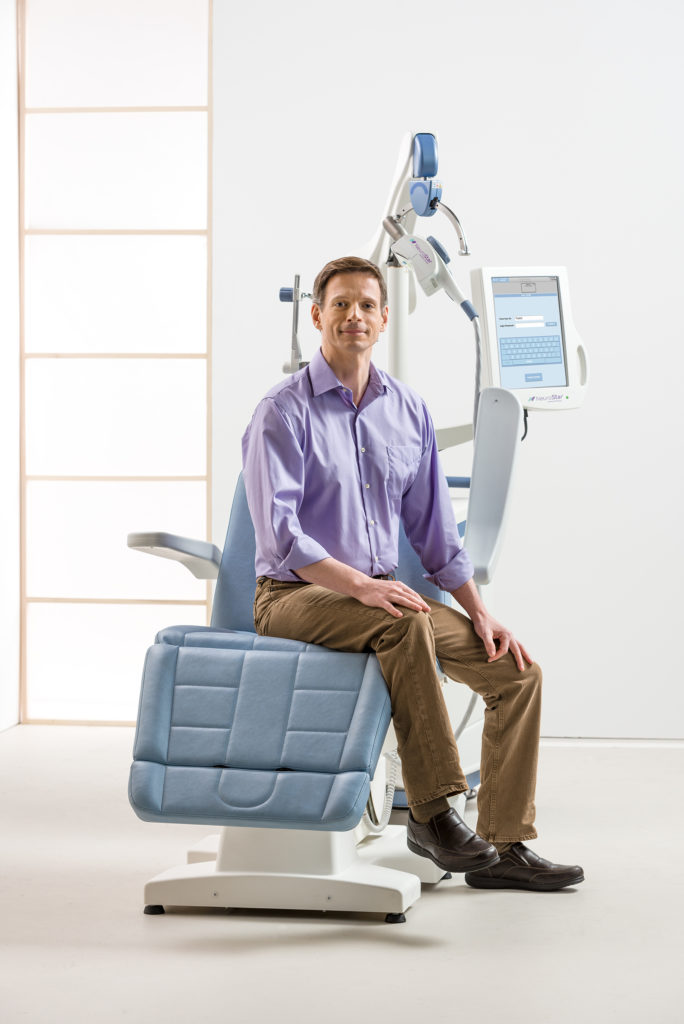
During a treatment session with Apollo, a magnet similar to those used in a magnetic resonance imaging (MRI) machine is used to stimulate key areas of the brain. These areas are believed to hold mood-controlling nerve cells, and the magnetic pulses may positively affect the brain’s neurotransmitter levels. This treatment makes long-term remission possible.
Treatment with Apollo Advanced Therapy is easy:
- Apollo therapy sessions are conducted at the Holiner Group office
- You can resume normal life activities immediately
- You are awake during the entire treatment
- There are no negative effects on memory or sleep
- Most health insurance plans cover it, including Medicare and Tricare